Background: Protein C is a zymogen that suppresses the coagulation cascade through proteolysis of factors V and VIII, and therefore its deficiency is a risk factor for thromboembolic events 1. There are two main subtypes of protein C deficiency. In type I protein C deficiency, both protein C antigen and activity levels are decreased, resulting in low protein C or inadequate protein lifespan 2. In type II deficiency, the activity level is lower than the antigen level due to abnormal protein activity 2. Inherited protein C deficiency is caused by either compound heterozygous or homozygous pathogenic variants in the POMC gene.
Objective: To describe the clinical presentation and outcomes of 2 severe protein C deficiency cases.
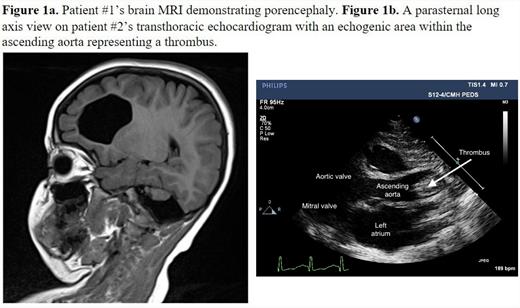
Patient #1 is an 8-year-old male with history of neonatal stroke, dense left hemiplegia, and cerebral palsy, who underwent whole exome sequencing due to MRI findings (Figure 1a). This identified an apparently homozygous variant in POMC, c.1154T>C
(p. Met385Thr)in exon 9. Both parents were confirmed to be heterozygous carriers. The patient was found to have a normal antigen of 62% and a severely low activity of <10%, suggesting a diagnosis of type II protein C deficiency. He is being considered for extended anticoagulation with a direct Xa or IIa inhibitor or a vitamin K antagonist.
Patient #2 was born at 34 weeks and presented immediately following birth with a large occlusive thrombus in the ascending to proximal aorta as well as left ventricular dysfunction and mitral regurgitation (Figure 1b). She was not a candidate for thrombectomy or catheter-directed tissue plasminogen activator (TPA) due to her age and procedural risks. She was given systemic TPA due to severe end organ dysfunction followed by unfractionated heparin (UFH). Due to neurological changes and intracranial hemorrhage (ICH), TPA and UFH were discontinued. Fresh frozen plasma and protein C concentrate were administered and UFH was resumed. Due to worsening ICH, UFH was again stopped, and she developed a left ventricular thrombus, impairing her systemic circulation. Her clinical status worsened, and she became unresponsive to resuscitation efforts, and passed away on DOL 16. Testing identified a protein C antigen and activity level of 10% and <15% respectively. This patient was found to have 2 variants in the PROC gene, c.30C>T (p. Phe10Phe), which is a variant of uncertain significance, and c.1019C>T (p. Thr340Met), which is known to be pathogenic.
Discussion: Both cases highlight uncommon presentations of severe protein C deficiency without purpura fulminans. Management of severe protein C deficiency consists of protein C replacement or long-term anticoagulation with warfarin as the standard of care 3,4. However protein C concentrate is costly, requires intravenous access, and can cause hypersensitivity reactions and hemorrhage. Long-term warfarin treatment is burdensome for families, requiring frequent laboratory monitoring with variable time in the therapeutic range. The only curative option for protein C deficiency is liver transplantation, which has additional risks and has rarely been reported in the literature 3,4.
Conclusions: These cases illustrate the phenotypic heterogeneity of inherited severe protein C deficiency and demonstrate the challenges of clinical management in this patient population. The International Society on Thrombosis and Haemostasis initiated a severe protein C deficiency registry in 2013 which will standardize and improve treatment of this rare condition.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal